
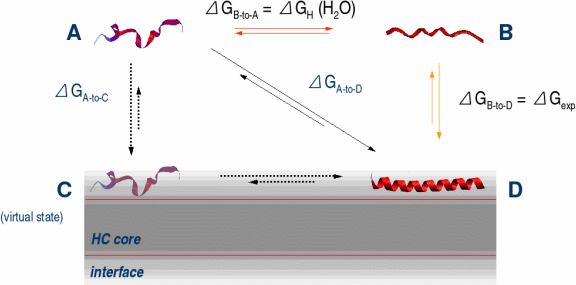
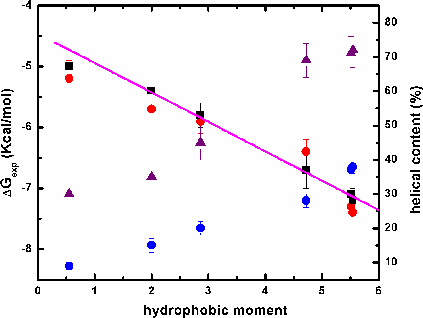
Amphipathic helical peptides bind strongly to membranes. But what is the role of amphiphilicity in interfacial partitioning, and how can it be quantitated? In order to answer these questions, we synthesized two series of peptides: In the first we changed the content of hydrophobic amino acids, and in the second we kept it constant while changing the hydrophobic moment, μH. We started with Ac-(AAQAA)3-GW-NH2, μH = 1.7 (A12Q3-1.7) and gradually increased hydrophobicity by Ala-to-Leu substitutions and produced amphipatic and non-amphipatic amino acid distributions. Only the model peptide, A8L4Q3-4.7, with a high μH of 4.7 bound significantly to POPC membranes. Changing the μH from 2 to 4.7 appears to improve the free energy of transfer by ~5 kcal mol-1. Therefore, amphilicity is indeed important. The second series of peptides was studied using CD and fluorescence spectroscopy to establish a relationship between μH, partitioning and helicity. Our results show that (1) the helical content in water and in the membrane increases with μH and (2) the total free energy of binding to the membrane decreases with μH. The free energy per residue of helix formation on the membrane is -0.3 kcal mol-1 per residue and it is the same for all peptides. Previously, we showed the absence of complete additivity of hydrophobic and electrostatic interactions using a series of cationic peptides [Ladokhin and White, (2001) JMB, 309, 543-552]. Because the peptides in the current study bear no charge, the free energy of partitioning into both neutral and anionic vesicles can be compared directly, without interference from Coulombic interactions. We found no difference in the binding to either type of membranes, suggesting that there is no influence of charge on ΔGμH.
| Name | Sequence | *μH | *,**ΔG (kcal/mol) |
|---|---|---|---|
| * Values calculated using the program MPEx. | |||
|
** Calculated from Wimley-White hydrophobicity scale
[Wimley and White, (199 6) Nature Struct. Biol. 3, 842-848] and corresponds to ΔGA-to-C in the thermodynamic cycle. |
|||
| A8L4Q3 -0.55 | Ac-LQALAAQALQAAALAGW-NH2 | 0.55 | -3.44 |
| A8L4Q3 -2.00 | Ac-LAQAAALQLLAAQAAGW-NH2 | 2.00 | -3.44 |
| A8L4Q3 -2.86 | Ac-AQLAALAALQAAQLAGW-NH2 | 2.86 | -3.44 |
| A8L4Q3 -4.72 | Ac-AAAQAAAQLLQALLAGW-NH2 | 4.72 | -3.44 |
| A8L4Q3 -5.51 | Ac-QLAQALAAALAALAQGW-NH2 | 5.51 | -3.44 |
| A8L4Q3 -5.54 | Ac-QALQALAAALAALAQGW-NH2 | 5.54 | -3.44 |
|
|
|
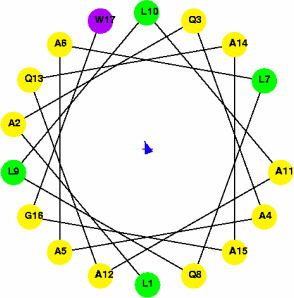
μH = 2 (non-amphipathic) |
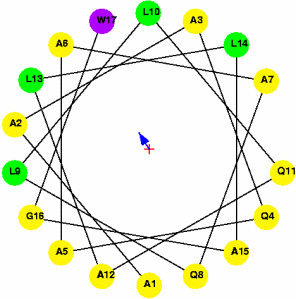
μH = 4.72 (amphipathic) |
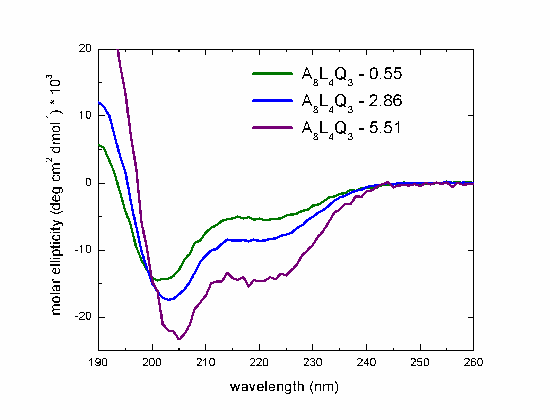
CD spectra in water of the peptides with hydrophobic moment of 0.55, 2.86 and 5.51, measured at 298K.
| Name | *,**[Θ]222nm | %Helix | *,***[Θ]222nm | %Helix |
|---|---|---|---|---|
| * Units of [Θ] are deg cm2dmol-1. | ||||
| ** Values of molar ellipticities in water. | ||||
| *** Values of molar ellipticities in the membrane. | ||||
| A8L4Q3 -0.55 | -4400 | 9% | -12000 | 30% |
| A8L4Q3 -2.00 | -6500 | 15% | -13000 | 35% |
| A8L4Q3 -2.86 | -8200 | 20% | -16000 | 45% |
| A8L4Q3 -4.72 | -10700 | 28% | -24000 | 69% |
| A8L4Q3 -5.51 | -13500 | 37% | -24700 | 71% |
| A8L4Q3 -5.54 | -14000 | 38% | -25000 | 72% |
Some of our peptides have Leu pair interactions (i, i+3) and (i, i+4) which stabilize the helices [Luo and Baldwin, (2002) Biophys. Chem., 96, 103]. Applying MPEx to their peptides, we observed the same effect: Increasing the μH increases the helicity:
| Name | Side-Chain Interaction | %Helix | μH |
|---|---|---|---|
| Ac-KAAAAKAALAKLAAAKGY-NH2 | (i, i+3> | 26% | 0.57 |
| Ac-KAAAAKAALAKALAAKGY-NH2 | (i, i+4) | 33% | 1.33 |
| Ac-ELAALKAKLAALKAKAGY-NH2 | 2(i, i+3) + (i, i+4) | 43% | 1.48 |
| Ac-ELAALKAKLAALKAKLGY-NH2 | 2(i, i+3) + 2(i, i+4) | 51% | 1.62 |
|
|
|
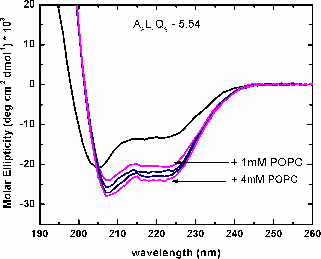
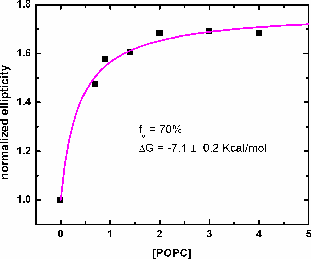
| CD spectra of A8l4Q3 - titrated with POPC LUV. Increasing the concentration of lipid increases the helical content, measured as molar ellipticity at 222nm. | Binding curve obtained from the titration of A8L4Q3 with POPC LUV. The binding curve gives the maximum helical content in the membrane and the binding constant. The free energy of Gibbs is calculated as: ΔG = -RTlnK. |
|
|
|
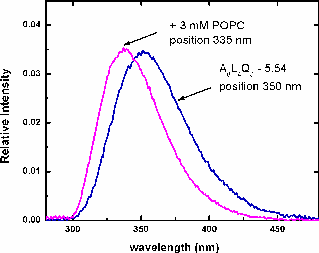
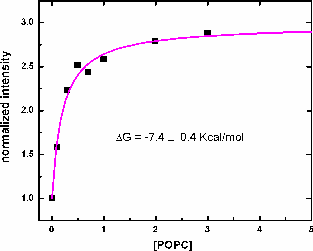
| Flourescence spectra of A8L4Q3 -5.54 with the maximum position at 350nm (corresponding to Trp in water). Adding 3mM POPC LUV, the position shifts to 335 nm, indicating binding to the membrane. | Binding curve of the titration with POPC LUV of A8L4Q3 -5.54. The binding constant and the free energy are obtained, and they are consistent with the values obtained by CD. |
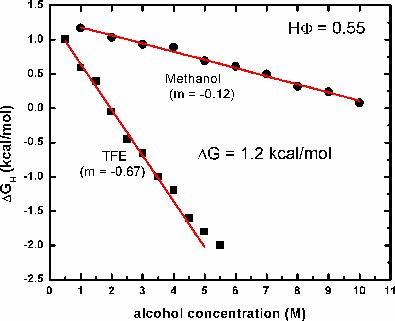
ΔGH = ΔGH(H2O) - m[alcohol] [Hirota et al., (1997) Protein Sci. 6:416-421):

This work was supported by NIH grants GM 46823 and GM 069783.